Occurs by regeneration and proliferation
Ø Some tissues are able to replace the injured cells and return to normal state this process is called as regeneration
Ø It occurs by the proliferation of uninjured cells (residual cells ) which having the capacity to divide and also by replacement from stem cells.
Ø In rapidly dividing epithelia of skin and intestines regeneration process is typical one.
Ø It occurs also in some parenchyma organs like liver.
Ø Some tissue perform the scar formation process
ØScar formation occurs when the tissues are unable to do the process of regeneration or if the supporting structure of tissues are damage severly
Ø Although scar formation can make the tissue able to perfom its function but it cant able the tissue to replace its parenchymal cells.
Ø Term fibrosis is often use to describe the collagen extensive collagen deposition, occurring in lungs, kidney and other organs as a result of chronic inflammation.
Ø If fibrosis occurs in a tissue space surrounded by inflammatory exudates then that is called as organization, for example, organizing pneumonia.
Proliferation
Ø In proliferation
following cells involves during tissue repair.
Ø The remnants of injured cells; which causes the
restoration of normal cells
Ø Vascular endothelial cells; which cause the formation of new blood vessels and provide nutrients for the repair process.
Ø Fibroblast cells; the source of fibrous tissue which causes
the filling of the defects by forming scars which cant be done by the
regeneration process
Ø The proliferation of the above three cells is
done by the growth factors
Ø The normal cells
pool/population is done the balance proliferation among cells, deaths
by apoptosis and formation of new differential cells in need.
Ø The key process in
the regeneration is DNA replication and mitosis.
Ø And the sequence of
events that control these processes is called as cell cycle.
Ø The non dividing
cells or permanant cells are in G1 phase which is in cell cycle arrest or can
exite the cycle coming to the G not phase,
Ø So growth factors
can take from G not to G1 and then gradually to the s phase which is growth
phase
Ø Once cell enter the
S phase their DNA become replicated and then progress through G2 and mitosis.
Ø The ability of
tissues to proliferate themselves is typically determined or influenced by their
intrinsic proliferative capacity.
Ø On the basis of this
proliferative capacity body tissues is divided into three types.
1)Labile
tissues (continuously dividing);
Ø Cells of these
tissues are lost and replaced continuously
Ø They are replaced
and mature from stem cells and then these mature cells are proliferated
Ø As long as the stem
cells are present these cells will be readily regenerated after injury.
Ø For example
HSC, stratified squamous epithelium of cervix, vagina, oral cavity, and skin.
Ø The cuboidal
epithelium of the ducts draining the
exocrine glands
Ø The columnar
epithelial of git, uterus,fallopian tube. And transitional epithelial of the urinary tract.
2) Stable /quiescent tissues;
Ø Quiescent mean nonactive so cells of these tissues are not active and have less replicting
activity in their normal state.
Ø But they can
replicate in response to injury or loss of tissue mass
Ø They are composed of
the parenchyma of the most solid tissues of the body like the kidney, liver, and
pancreas.
Ø They also include
endothelial cells, fibroblast, and smooth muscle cells
Ø Their proliferation
is important in wound healing
Ø With exception of the liver stable cells have a limited capacity for regeneration.
Ø Only stable and labile tissues can regenerate.
3)Permanent
tissues;
Ø These tissues are composed of those cells who dont have ability to proliferate
Ø These includes the
heart and composed o neurons and cardiac muscle cells
Ø Once they can get
injured they cant be regenerates and thats why injury to the brain will be
result on scar formation
Ø In permanant tissues
repair is dominant by scar
Ø In short we can
say that two things are required for
regeneration
1) stem cells
2) Uninjured remnants of damage tissue (from which proliferation will occur)
Ø And if stem cell or
uninjured cells are not present then tissue goes for repairing through scaring.
Ø A point worthy of emphasis is that extensive regeneration or compensatory hyperplasia can occur only if the residual connective tissue framework is structurally intact, as after partial surgical resection. By contrast, if the entire tissue is damaged by infection or inflammation, regeneration is incomplete and is accompanied by scarring.
Scar
formation
Ø If the tissue injury
is severe or chronic that it causes the destruction of parenchyma, epithelial, and connective tissues.
Ø Under these
conditions repair occurs by the deposition of connective tissue upon the nonregenerated
cells which lead to the formation of scar.
Steps in scar formation;
· Formation of new blood vessels or angiogenesis
· Migration and
proliferation of fibroblast &
and deposition of connective tissues,
which then together with the preformed
abundant vessels and in between leukocytes
there.
· this tissue now has the pink granular
appearance and is called granulation
tissue.
· Maturation and reorganization of the fibrous tissue or remodeling to produce a stable fibrous scar.
Overview of scar formation;
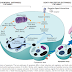
Ø Repair begins within 24 hours of injury with
the emigration of fibroblast cells and their induction and proliferation of
endothelial cells
Ø After 3 to 5 days tissue granulation occurs which is a characteristic sign of healing.
Ø The term granulation
derives from the gross appearance such as that beneath the injured scab of
skin.
Angiogenesis
Ø The process of formation of new blood vessels from existing ones primarily venues
Ø It consists of the following steps.
· Vasodilatation in
response to NO and increase permeability in response to VEGF.
· Separation of
pericytes from the outer surface of blood vessel
· Migration of
endothelial cells toward the site of injury
· Proliferation of endothelial
cells
· Remodeling into capillary
tubes
· Recruitment of
pericytes for small capillaries and smooth muscle cells for larger vessels to form
a mature vessel.
· Suppression of endothelial cells proliferation and migration
Ø Angiogenesis involves some growth factors, cell to cell interactions, and interaction with ECM proteins, and tissue enzymes.
Growth factors in angiogenesis;
Ø Several growth
factors are involved but the most important are VEGF and FGF2
Ø VEGF family:
Ø They are the most important ones are composed of
VEGF, A, B, C,D, and E& PIGF.
Ø Vegf a is also
called VEGF its an important and major inducer of angiogenesis after injury
Ø VEGF b and PIGF
involves in vessel formation in the embryo
Ø VEGF c and d
stimulate both lymphangenensis (lymphatic vessels) and angiogenesis.
Ø VEGFS are stimulated
in most adult tissues but the highest expression in endothelial cells
Ø They bind to a
family of tyrosine kinase receptors (VEGFR 1,2 and 3).
Ø Most important it for angiogenesis is VEGFR 2.
Ø There are many
inducers of VEGF most important one is hypoxia
Ø Vegfs cause
migration and proliferation of endothelial cells
Ø The capillary
sprouting process is initiated by the VEGF.
Ø it promotes the
vasodilation by stimulating the NO
Ø and contribute to the formation of the vascular lumen.
Ø Antibodies against VEGF
are approved for the treatment of some tumors that depend on angiogenesis for
their spread and growth. These antibodies are also used in the treatment of
“wet” (neovascular).
Ø FGF-2 participates in
angiogenesis mostly
by stimulating the proliferation of
endothelial cells. It also promotes the migration of macrophages and fibroblasts to the damaged area and
stimulates epithelial cell migration to
cover epidermal wounds.
Ø Angiopoietins Ang1 and
Ang2 are
growth factors that play a role in angiogenesis and the structural maturation
of new vessels
Ø Newly formed vessels
need to be stabilized
by the recruitment of pericytes and smooth muscle cells and by the deposition
of connective tissue.
Ø
participate in the stabilization process—PDGF recruits smooth muscle cells and TGF-β suppresses endothelial proliferation
and migration, and enhances the production of ECM proteins
ECM proteins
Ø ECM proteins participate
in the process of vessel growing in angiogenesis
Ø largely through
interactions with integrin receptors in endothelial cells and by providing the platform
for vessel growth
Ø Enzymes in the ECM,
notably the matrix metalloproteinases (MMPs), degrade the ECM to permit
remodeling and extension of the vascular tube.
Ø Newly formed vessels
are leaky because of incomplete interendothelial junctions and because VEGF
increases vascular permeability.
Activation of fibroblast and deposition of connective
tissues.
Ø The laying down of
connective tissue in the scar occurs in two steps: (1) migration and
proliferation of fibroblasts into the site of injury and (2) deposition of ECM
proteins produced by these cells
Ø The recruitment and
activation of fibroblasts to synthesize connective tissue proteins are driven
by many growth factors, including PDGF, FGF-2, and TGF-β.
Ø The major source of
these factors is inflammatory cells, particularly macrophages, which are
present at sites of injury and in granulation tissue.
|
primary intention. |
Secondary intention. |
|
A small scar is formed,
but |
When cell or tissue loss
is more extensive, the
repair process is more complex and involves a combination of |
|
24 hours, neutrophils are seen at the incision margin,
migrating toward the fibrin clot. |
A larger clot or scab rich in fibrin
and fibronectin forms at the surface of the wound |
|
Within 24 to 48 hours,
epithelial cells from both edges have begun to migrate and proliferate along with the dermis, |
Larger defects require a
greater volume of granulation tissue to fill in the gaps and provide the
underlying framework for the regrowth of tissue epithelium. A greater volume of granulation tissue
generally results in a greater mass of scar tissue. |
|
By day 3, Collagen
fibers are now |
Secondary healing
involves wound contraction. Within 6
weeks, for example, large skin
defects may be reduced to 5% to 10% of their original size, largely by
contraction |
|
day 5, neovascularization reaches its peak as granulation |
|
|
During the second week, there is continued
collagen |
|
|
By the end of the first month, the scar consists of a cellular connective
tissue, largely devoid of inflammatory |
|
- Wound healing can be altered by many conditions, particularly infection and diabetes; the type, volume, and location of the injury are also important factors in healing.







0 Comments